Lewis Structure Of Po3 1 20 Printable Electronegativity Periodic Table Trend Forms And. Trends in electronegativity down a group.

Periodic Trends Made Easy Chemtalk
Why is this part of the periodic trends.
. Periodic Trends and Patterns 1. Electronegativity usually rises from left to right. We can see this with the help of a graph showing the trend in electronegativity in period 3 from sodium to chlorine.
On the Periodic Table of elements electronegativity increases as you move left to right across a period. Periodic trends are patterns in elements on the periodic table. Trends in electronegativity are reminiscent of our trend in atomic radius and for a good reason.
It doesnt have an electronegativity because it doesnt form bonds. As you move from left. The trends for electronegativity is that the value increases across the periods rows of the periodic table.
Atoms whose nuclei pull the hardest on their own outer electrons should by reason pull on shared electrons in their chemical bonds hardest as well. This allows a stronger attraction between outermost electrons and the nucleus. Electronegativity follows patters similar to that of an atoms ionization energy and electron affinity.
The highest electronegativity value is for fluorine. It gradually increases when moving up the periodic tableThis occurs because it is gaining electrons from the covalent bonds it holds with other atoms. Trends in electronegativity across a period.
Electronegativity measures an atoms tendency to attract a bonding pair of electrons. So electronegativity increases up a group and across the row of the periodic table. The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner.
Electronegativity increases from left to right of a row as across the row the number of protons in the nucleus increases. It sees a decreasing trend when you move down a group. What is period trend for electronegativity.
Major trends are electronegativity ionization energy electron affinity atomic radius and metallic character. 1 Development of the Modern Periodic Table MAIN Idea The periodic table evolved over time as scientists discovered more useful ways to compare and organize the elements. Since the electronegativity of some of.
Electronegativities generally decrease from top to bottom of a group. Across a period in the periodic table. Periodic Table Trends.
Electronegativity is the ability of an atom to pull electrons towards it. Decrease in a group down and increase in a period from left to right. 2 hours agoB there is a general trend in ionization energy down the periodic table.
The chart shows electronegativities from sodium to chlorine - you have to ignore argon. Explore electronegativity and its trends among groups and periods in the periodic table and discover why some. In this graph we have not shown argon as it does not react with elements to form bonds.
The existence of these trends is due to the similarity in atomic structure of the elements in their group families or periods and because of the periodic nature of elements. Which of the following trends is similar to electronegativity follows the same pattern. Identify the patterns of electronegativity on the PT and explain why these patterns make sense with the valence electrons of the groups.
Predict the properties of elements from their position in the Periodic Table given relevant information and to identify trends in other groups of elements. Electronegativity values generally increase from left to right across the periodic table. As you go across a period the electronegativity increases.
The trends of the electronegativity are. As you go down a group electronegativity decreases. Lithium 10 and Fluorine 40 in period 2.
In general electronegativity decreases as you move down a group in the periodic table this correlates. Refer to your completed periodic tables to discuss the. 9th - 12th grade.
For example the electronegativity trend across period 3 in the periodic table is depicted below. As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table. The electron setup or organization of electrons circling neutral particles demonstrates a common example of periodicity.
It shows how an atom can swiftly form a chemical bond. As mentioned the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements. When moving from left to right across the periodic table electronegativity increases with the exception being the noble gases.
Make circles with the diameter listed. Periodic Table Trends. The electronegativity also increases up a group column of the periodic table.
Trends in electronegativity across a period. The electrons involve a progression of electron shells numbered 1 2 etc. These trends are readily.
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. However there are exceptions to it at times. In cm while the actual values for ionization energy is in joules.
When we move from left to right in a period of the modern periodic table electronegativity increases. Periodic Trends in the Electronegativities of Elements. Lithium 10 and Francium 07 in Group I.
People also asked Of elements in. Make lines from the bottom of each box that are the appropriate length according to the data size follows the trend that it does. Each shell comprises at least one subshell named s p d f and g.

Electronegativity Definition And Trend

Periodic Trends In Electronegativity Ck 12 Foundation
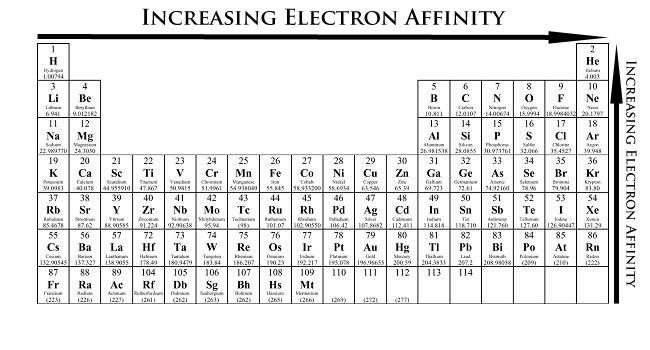
Periodic Trends Chemistry Libretexts

Electronegativity Definition And Trend

What Is Electronegativity Trends Chart Periodic Table Chemtalk
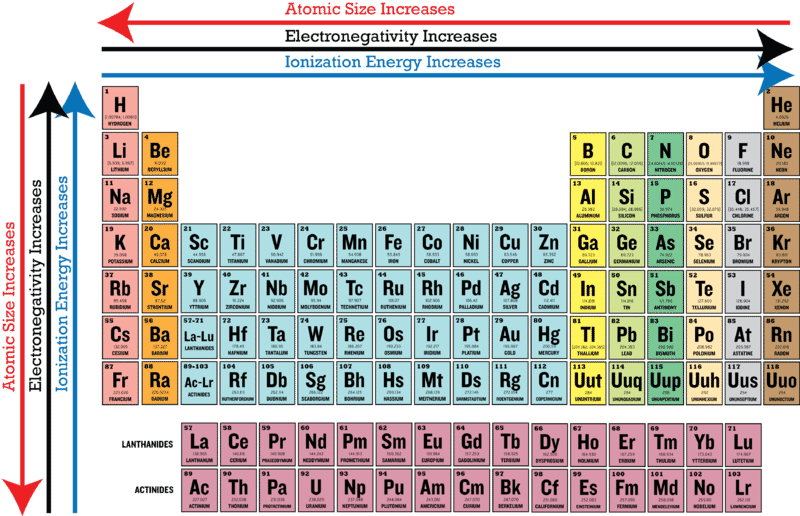
Periodic Trends In Electronegativity Ck 12 Foundation
What Trend In Electronegativity Do You See As You Go Across A Period Row On The Periodic Table Socratic
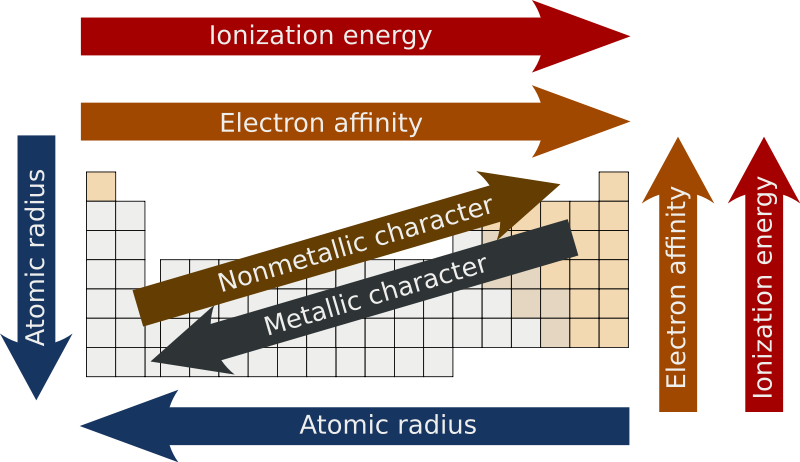
0 comments
Post a Comment